Categories
Tags
Share:
More Articles
-
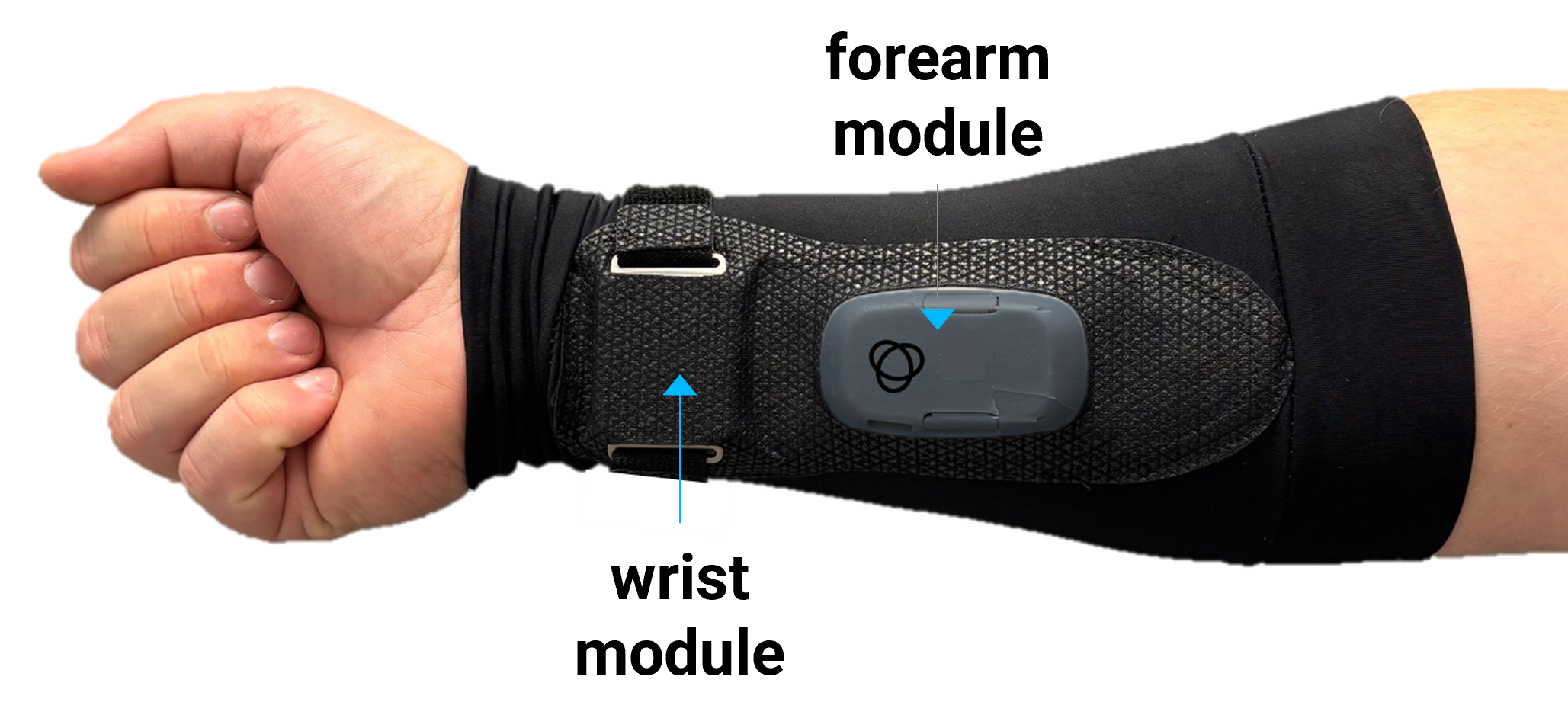
Innovation at the Intersections: How Luna Labs Creates Breakthrough Solutions for Patients and Warfighters
What makes Luna Labs different in biomedical innovation? Biotech Division managers, Lauren Costella and Kelley Virgilio, reveal the company's unique approach to tackling complex biotech challenges.
Categories
Tags
-
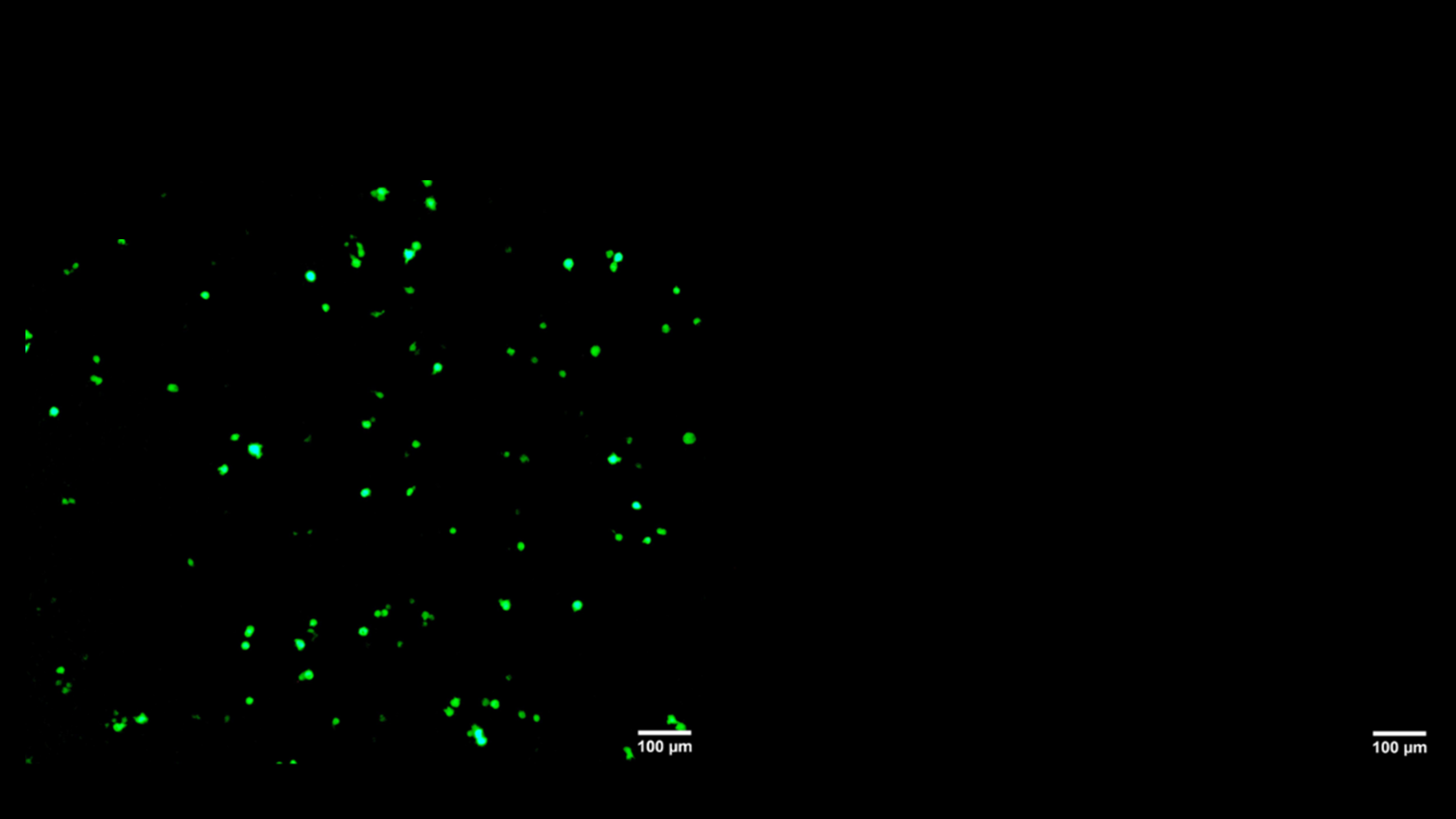
A More Effective Way to Deliver Therapeutics Across the Body’s Natural Barriers
NanoVac is a delivery platform that mimics virus structure to navigate the body's defenses and deliver drugs more effectively.
Categories
Tags
-

Excitement Builds for 2026: Latest News from Luna Labs’ Acuity Corrosion Team
Reflecting on Acuity 2025 highlights and preparing for another meaningful year of connection and innovation.
Categories