


Acceleration of mRNA Therapies
The importance of rapidly developing effective vaccines in response to emerging pathogens was demonstrated in the COVID-19 pandemic, with over 12 billion doses administered globally.
The current delivery method for mRNA vaccines, including for COVID-19, is lipid nanoparticles. They have known inflammatory reactions and are not designed to enhance the innate immune response.
In response to the need for flexible vaccine and therapeutic delivery technologies, Luna Labs has been developing delivery vehicles for mRNA and proteins since 2015.
NanoVac™ is the company’s short carbon nanotube-based platform that can safely and efficiently deliver a broad range of antigens and therapeutics via multiple administration routes.
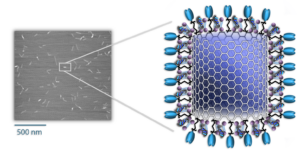
An electron microscope is required to see the NanoVac delivery vehicle, with each rod 100-200 nm long. In the schematic, the loading of nucleic acids and proteins on the surface of the vehicle is illustrated.
Vaccination Against HIV-1
In collaboration with New York University and Columbia University, Luna Labs developed mRNA and protein-based HIV-1 vaccines and demonstrated their efficacy in humanized mouse models.
By mimicking virion size and optimizing its antigen, NanoVac is more effective at vaccinating compared to standard lipid nanoparticle-mRNA delivery.
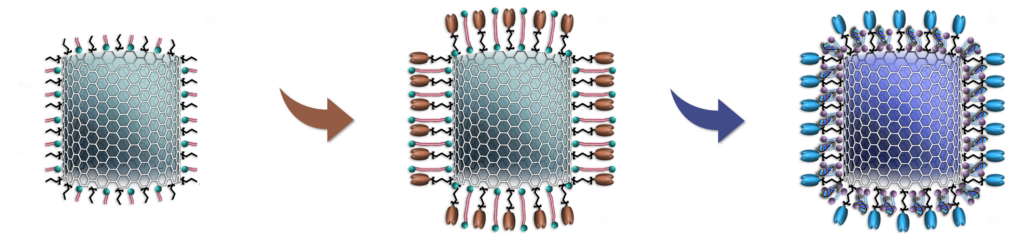
NanoVac Vehicle
- Mimics virus size
- Supports biocompatibility and circulation
NanoVac Vaccine
- Can be immobilized with proteins, mRNA, or both
- Allows control of antigen density and epitope presentation
NanoVac
- Current development for HIV-1 vaccination
- Both mRNA and V1V2 protein subunits delivered
Applications
The NanoVac platform is uniquely suited for addressing global health concerns and has potential integration into wider clinical applications to fight cancer, deliver therapeutics and more.
There are over 180 mRNA-based agents in pharma companies’ pipelines, and 80% of them are in the preclinical stage.
NanoVac provides an exciting opportunity to enhance their delivery, expand their method of administration beyond the intramuscular route, and reduce known inflammatory side effects with existing vehicles.
- Engineered to vaccinate against multiple targets simultaneously.
- Controls antigen presentation and enhances cellular uptake.
- Mimics virion size and shape to stimulate antibody production.
- Reduces unwanted inflammatory reactions seen with lipid nanoparticles used in current mRNA delivery.
- Stablizes mRNA and proteins, enabling cold-storage flexibility.
- Compatible with intramuscular and intranasal applications.
- Easily produced with widely available and low-cost components.
More Information
Contact us with questions or for partnering opportunities.
